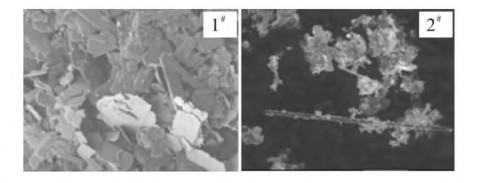
本文以2种不同形貌升华氧化钼为原料,采用TG-DTA法研究了不同升温速率(3℃/min、5℃/min、10℃/min)下氢气还原氧化钼的动力学。结果表明:2种不同形貌的升华氧化钼一段还原反应起止还原温度几乎相同。但失重速率480℃前1#MoO3高于2#MoO3,480℃后失重速率低于2#MoO3。二段还原反应1#MoO3的反应温度较2#MoO3低。再通过用Flynn-Wall-Ozawa和Kissinger动力学分析方法计算2种不同形貌升华氧化钼的一段平均表观活化能为为89.26,2#MoO3为79.86 kJ/mol,而二段表观活化能1#MoO3平均为103.53 kJ/mol ,2#MoO3为93.16 kJ/mol。
In this paper, two different morphologies of sublimated molybdenum oxide are used as raw materials, and the kinetics of hydrogen reduction of molybdenum oxide at different heating rates (3℃/min, 5℃/min, 10℃/min) are studied by TG-DTA method. The results show that the starting and ending reduction temperatures of the two different morphologies of sublimated molybdenum oxide one-stage reduction reaction are almost the same. But the weight loss rate of 1#MoO3 is higher than 2#MoO3 before 480℃, and the weight loss rate is lower than 2#MoO3 after 480℃. The reaction temperature of the second-stage reduction reaction 1#MoO3 is lower than that of 2#MoO3. Then by using Flynn-Wall-Ozawa and Kissinger kinetic analysis method to calculate the average apparent activation energy of two different morphologies of sublimated molybdenum oxide is 89.26, 2#MoO3 is 79.86 kJ/mol, and the second stage of apparent activation energy The average of 1#MoO3 is 103.53 kJ/mol, and the average of 2#MoO3 is 93.16 kJ/mol.