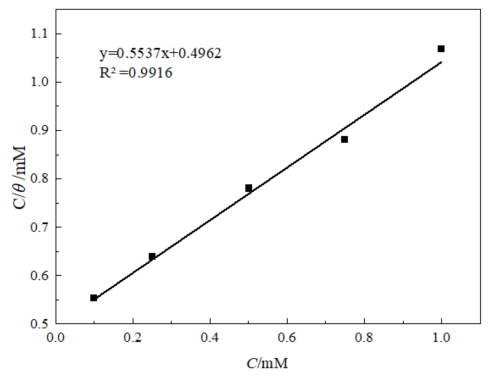
采用加入0.5%BQD缓蚀剂在90℃、20 wt.%盐酸中的失重评价数据,来研究其在盐酸中的吸附行为。吸附热力学是通过研究缓蚀剂的浓度C与浓度和覆盖度比值(C/θ)之间的关系曲线,并对得到的关系曲线进行拟合,确认最符合的等温吸附模型来计算相关的吸附热力学参数。
The data from the weight loss evaluation of the addition of 0.5% BQD corrosion inhibitor in hydrochloric acid at 90°C and 20 wt.% hydrochloric acid were used to study its adsorption behavior in hydrochloric acid. The adsorption thermodynamics was calculated by studying the relationship curve between the concentration C of the corrosion inhibitor and the ratio of concentration and coverage (C/θ) and fitting the obtained relationship curve to confirm the most compatible isothermal adsorption model to calculate the relevant adsorption thermodynamic parameters.