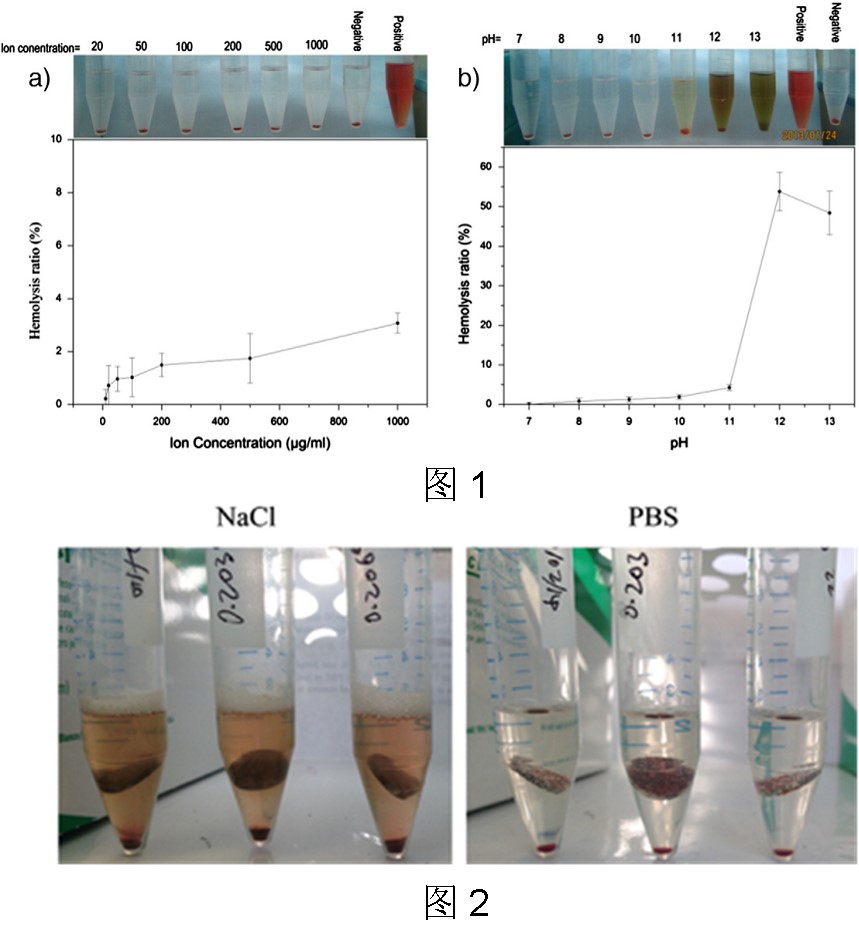
图1-a显示了不同Mg2+浓度的溶血率。 可以发现,溶血率随着Mg2+浓度的增加而增加,但即使Mg2+浓度达到1000 μg/mL时,溶血率仍低于5%。 Mg2+ 过量导致溶血抑制,可能是因为总离子强度显着增加。 随着Mg2+浓度的增加,溶液的渗透压会增加。 这些压差会导致红细胞肿胀和细胞膜破裂,释放出游离血红蛋白,从而导致红细胞溶血。图1-b显示了不同pH值的生理盐水中的溶血率,从中可以看出pH值对溶血的影响很大。 当 pH < 10 时,未发现溶血。 但在 pH = 12 时,溶血率飙升至 53.8 ± 4.8%。 如图2所示,在溶血试验中,高pH溶液(pH > 11)的颜色中变为棕黄色,而低pH溶液(pH < 11)的颜色保持原始颜色。由此可知,从红细胞中溶出的血红蛋白在高pH条件下变性,含铁元素的金属蛋白的中的铁原子将以Fe3+的形式离解,呈棕黄色。溶血试验测试的分光光度计比色法的机理是检测从破坏的红细胞释放的血红蛋白在540nm处的吸光度。一旦血红蛋白变性,它将在离心的底部沉淀。由于溶血试验仅测定上清液的吸光度,因此将排除变性沉淀的血红蛋白,这导致吸光度值低于实际值。另一方面,Fe3+也可能在540nm处有吸收,这也会影响实验结果。根据ASTM F756-00,在常规采用的溶血率测试方法中,盐溶液中Cl-的存在会造成镁合金产生严重的腐蚀,pH值产生较大变化,进而导致溶血率升高。为保证pH值的稳定,推荐采用缓冲液(如PBS)并在动态环境中进行溶血率测试。
Fig. 1-a shows the hemolysis ratio of different Mg2 + concentration. It can be found that the hemolysis ratio increases as the Mg2 + concentration increases, but is still lower than 5% even when the concentration of Mg2 + reaches 1000 μg/mL. The excesses of Mg2 + resulted in inhibition of hemolysis, probably because of the significant increase in total ionic strength. As Mg2 + concentration increases, the osmotic pressure of the solution will be enhanced. These pressure differentials can cause erythrocyte swelling and cell membrane rupture with release of free haemoglobin which could lead to the hemolysis of red blood cells. Fig. 1-b displays the hemolysis ratio in normal saline with diverse pH values, from which it can be seen that the pH value has a great effect on the hemolysis. When pH < 10, no hemolysis has been found. But the hemolysis ratio soars to 53.8 ± 4.8% at pH = 12. Results show that pure Mg in normal saline (NaCl) induces high hemolysis, of which the hemolysis ratio is 47.8%. On the contrary, in PBS pure Mg causes only 4.4% hemolysis as distributed in Fig. 2. The Mg2+ concentrations in the normal saline and PBS test systems are 25.93 and 9.23 μg/mL respectively, which are both less than 50 μg/mL. According to the results of Fig. 1-a), such a low Mg2+ concentration would not lead to a high hemolysis ratio. At the same time, though the pH values of the two test systems are below pH 11, of which is 10.72 in normal saline and 9.37 in PBS, it is the real cause for the high hemolysis. For the first reason, the corrosion product magnesium hydroxide is not hemolytic, so the corrosion surface of pure Mg could not induce the hemolysis. For the second reason, the test system is static that the great amount of OH
− ions produced in the corrosion reaction will remain and gather on the surface of Mg samples rather than uniformly distributed in the test solution. This means that the pH values of the solution around the magnesium samples will be higher than 11 or even 12, and the excessive increase of pH leads to instability of the membrane of the erythrocyte and results in the erythrocyte damage.