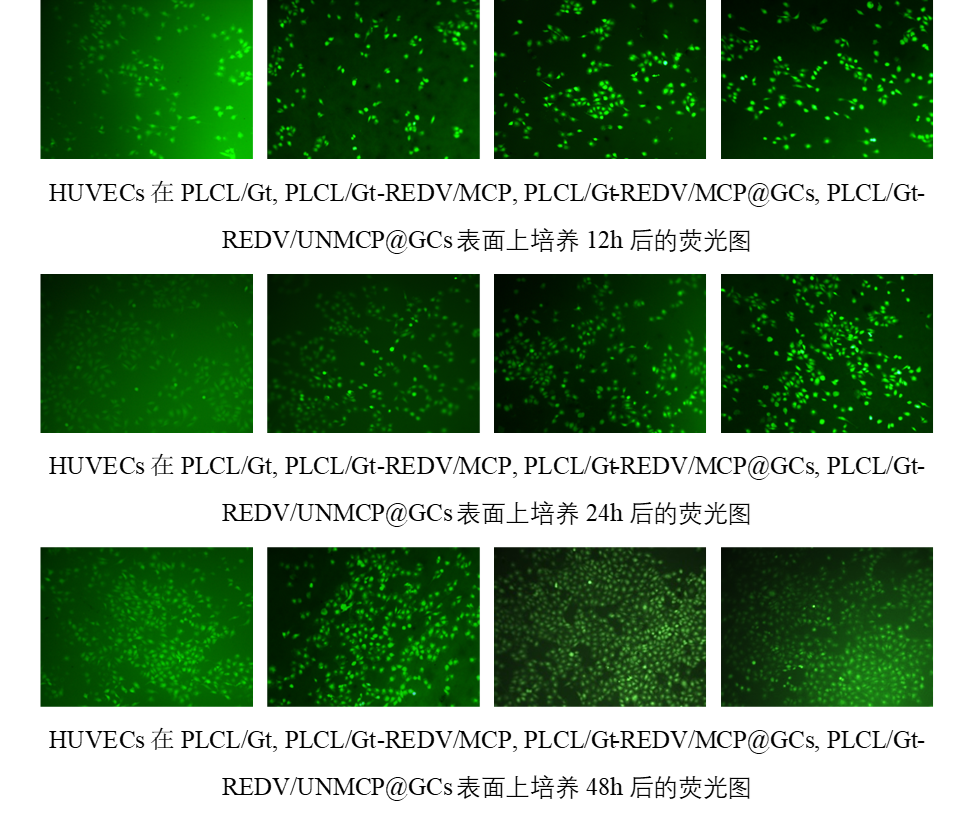
随着培养时间的增加,HUVECs在不同的膜上生长和增殖情况良好,表明基于PLCL/Gt的纳米纤维膜适合HUVECs的粘附和生长。在相同的孵育时间里,PLCL/Gt组的细胞密度略低于其他组,这主要归因于PLCL/Gt表面没有REDV粘附肽,而表面具有REDV的其他组可以促进HUVECs的粘附。此外,PLCL/Gt-REDV/MCP@GCs组的HUVECs密度在24和48 h显着高于其他组,表明HUVECs通过分泌MMP酶诱导MCP连接链的裂解,并触发释放GCs用于高效转染HUVEC。此外,PLCL/Gt-REDV/UNMCP@GCs表面上的HUVECs密度几乎与PLCL/Gt-REDV/MCP组相似,主要是因为GPQIGWGQ肽在HUVEC存在下是稳定的,该组中GCs是通过共价连接方式固定在该表面上而无法从表面释放。上述结果表明,PLCL/Gt-REDV/MCP@GCs表面能够有效促进内皮细胞的黏附和增殖。
HUVECs grew and proliferated well on different membranes with the increase of culture time, indicating that PLCL/Gt-based nanofiber membranes were suitable for HUVECs adhesion and growth. At the same incubation time, PLCL/Gt group showed slightly lower cell density than other groups since PLCL/Gt surface had non REDV adhesive peptide. Other groups having REDV on surface can promote HUVECs adhesion and proliferation. This primarily attributed to HUVECs selective adhesion effect of REDV peptide. Besides, HUVECs density of PLCL/Gt-REDV/MCP@GCs group was significantly higher than other groups at 24 and 48 h, indicating that HUVECs induced the cleavage of MCP linker via the enzymatic reaction of MMP on cell membrane and triggered to release GCs for highly efficient transfection of HUVECs. Furthermore, HUVECs density on PLCL/Gt-REDV/UNMCP@GCs surface was almost similar to PLCL/Gt-REDV/MCP group, which also proved that the GCs were covalently linked on this surface. But these GCs could not be released from the surface because the GPQIGWGQ peptide linker was stable in the present of HUVECs. The above results demonstrated that PLCL/Gt-REDV/MCP@GCs surfaces can effectively promote the adhesion and proliferation of endothelial cells.