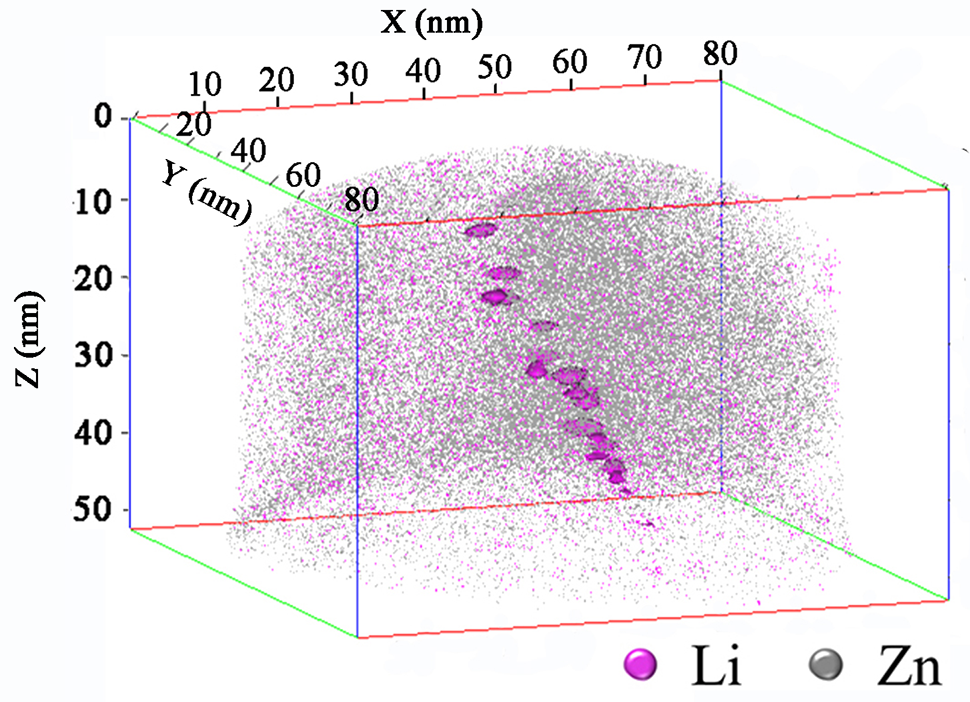
首次用3D-AP直接测定了Zn合金中的三维Li分布(图5(a))。贫锂锌基体中存在富锂区。据计算,贫锂锌基体中锂的浓度在0.1at.%左右波动,即0.01 wt%,较低温度下符合Zn-Li二元相图。因此,选择0.1 at% Li作为绘制等浓度面的标准。图5(a)三维重构后,在2.5×10−22m-3的体积内,18个富含Li的椭圆形析出相以0.1at.%Li的等浓度面突出。数字密度为7.16 × 1022m-3。长轴范围为2.6 ~ 6 nm,平均值为4.4±0.8 nm。代表性接近直方图如图5(b)所示。计算得到其核区Li与Zn的原子比为2/3,与Zn-Li二元相图中的α-Li2Zn3相一致(图1(a))。显然,这是一个亚稳态相,因为平衡相的组成应该是Zn+β-LiZn4。此外,对18种析出物的统计分析证实了锂锌比约为2/3。HRTEM进一步证实了亚稳态α-Li2Zn3析出相的存在。图5(c)中显示了一个直径为9.4 nm的粒子,图5(d)中红色实线包围的区域放大了。沿[0001]α投影的周期性点阵分布区域轴(如图5(d)所示)与α-Li2Zn3相的晶体结构(图1(b))一致。
Three-dimensional Li distribution has been determined directly in the Zn alloy by the 3D-AP for the first time (Fig. 5(a)). There are Li-rich regions in the Li-depleted Zn matrix. The concentration of Li in the Li-depleted Zn matrix is calculated to fluctuate around 0.1 at.%, i.e., equivalent to 0.01 wt.%, agreeing well with the Zn-Li binary phase diagram at lower temperatures. Therefore, 0.1 at% Li is chosen to be a criterion for drawing the isoconcentration surface. Within a volume of 2.5 × 10−22m-3after three-dimensional reconstruction in Fig. 5(a), eighteen Li-enriched elliptic precipitates Are highlighted by the isoconcentration surface of 0.1 at.% Li, with A number density of 7.16 × 1022m-3. Their long axes range from 2.6 nm to 6 nm, with an average value of 4.4 ± 0.8 nm.Representative proximity histogram is shown in Fig. 5(b). The atomic ratio of Li to Zn in its core region is calculated to be 2/3, in agreement with the α-Li2Zn3 phase in the Zn-Li binary phase diagram (Fig. 1(a)). Clearly, this is a metastable phase, since the equilibrium phase constitution should be Zn+β-LiZn4. Moreover, statistical analysis of the eighteen precipitates confirms that the Li to Zn ratio is around 2/3. HRTEM observations further confirm the existence of metastable α-Li2Zn3 precipitates.One with a diameter of 9.4 nm is shown in Fig. 5(c), and the region enclosed by the red solid lines is enlarged in Fig. 5(d).The periodic lattice distribution projected along the [0001] αzone axis(see the inset in Fig. 5(d)) agrees well with the crystal structure of the α-Li2Zn3 phase (Fig. 1(b)).