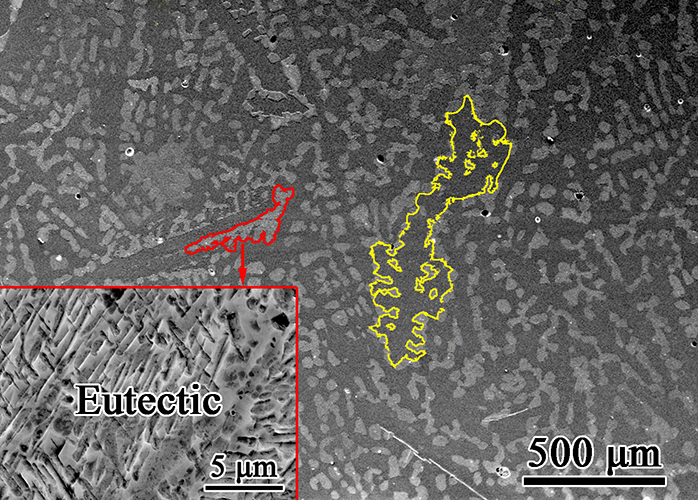
铸态合金的SEM图像如图2(a)所示,可以清楚地看到微观组织由较暗的组织组成更亮的区域。深色区域的面积占比为67.7%。根据Zn-Li二元相图,在凝固过程中首先形成初生的β-LiZn4相。根据杠杆定律计算,在403◦C的共晶温度下,理论密度为6.73 g/cm3的初生β-LiZn4相的体积分数为67.2%。计算的体积分数与测量的面积分数接近,因此图2(a)中较暗的区域应该是初生的β-LiZn4相,这是由于合金熔体中的晶体生长而形成的粗大的枝晶,这是各种合金中常见的现象。β-LiZn4枝晶的平均主臂长度为916±242 μm。根据锌-锂二元相图,锌+β-LiZn4 4共晶应该在初生的β-LiZn4枝晶之间形成,如图2(a)所示。插入图2(a)中较亮区域的放大图显示,它具有典型的共晶层状结构。共晶的长度范围为30 ~ 100μm。在图2(a)中还应该注意到,蚀刻后较暗的区域比较亮的区域低。Li(即-3.04 V)的标准电极电位(E◦)远低于Zn(即-0.76 V)。从图1(b)可以看出β-LiZn4的晶体结构类似于Li在Zn晶体中的固溶体。用Li原子取代Zn原子会使Zn的E◦更负,导致β-LiZn4的电偶腐蚀比Zn腐蚀快。由于Li在合金中的含量如此之低,很难直接检测到,因此其特征形貌有助于区分β-LiZn4和Zn。从图1(a)可以看出,在冷却过程中β-LiZn4中的Zn含量显著降低,导致Zn析出。图2(b)证实了这一点,在β-LiZn4基体中形成了致密的针状Zn沉淀。
SEM image of the as-cast alloy is shown in Fig. 2(a), in which.It can be seen clearly that the microstructure consists of darker and brighter regions. The area fraction of the darker region reaches 67.7%.According to Zn-Li binary phase diagram, primary β-LiZn4 phase first forms during solidification. Based on the lever rule, it can be calculated that the volume fraction of primary β-LiZn4 phase with a theoretical density of 6.73 g/cm3is 67.2% at the eutectic tem-perature of 403◦C. The calculated volume fraction is close to the measured area fraction, so that the darker region in Fig. 2(a) should be primary β-LiZn4 phase, which consists of coarse dendrites due to crystal growth in alloy melt, a common phenomenon observed in various alloys. The β-LiZn4 dendrites have an average primary arm length of 916 ± 242 μm. According to Zn-Li binary phase diagram, Zn+β-LiZn4 eutectics should form among the primary β-LiZn4 dendrites, which are
Confirmed in Fig. 2(a). An enlarged view of the brighter region in Fig.2(a) in the inserted figure reveals that it has a lamellar structure, typical for eutectics. The lengths of the eutectics range from 30μm to 100 μm. It should also be noted in Fig. 2(a) that the darker region is lower than the brighter region after etching. Standard electrode potentials (E◦) of Li (i.e. -3.04 V) is much lower than that of Zn (i.e., -0.76 V).It can be seen in Fig. 1(b) that the crystal structure of β-LiZn4 resembles a solid solution of Li in Zn crystal. The substitution of Zn atoms with Li atoms will make E◦of Zn more negative, so that β-LiZn4 corrodes faster than Zn due to galvanic corrosion. Since Li is difficult to be directly detected in such a low amount in the alloy, the characteristic topography is helpful to differentiate β-LiZn4 from Zn. According to Fig. 1(a), during cooling, Zn contents in β-LiZn4 decreases considerably, which will result in the precipitation of Zn.This is confirmed in Fig. 2(b), in which dense needle-like Zn precipitates form inβ-LiZn4 matrix.