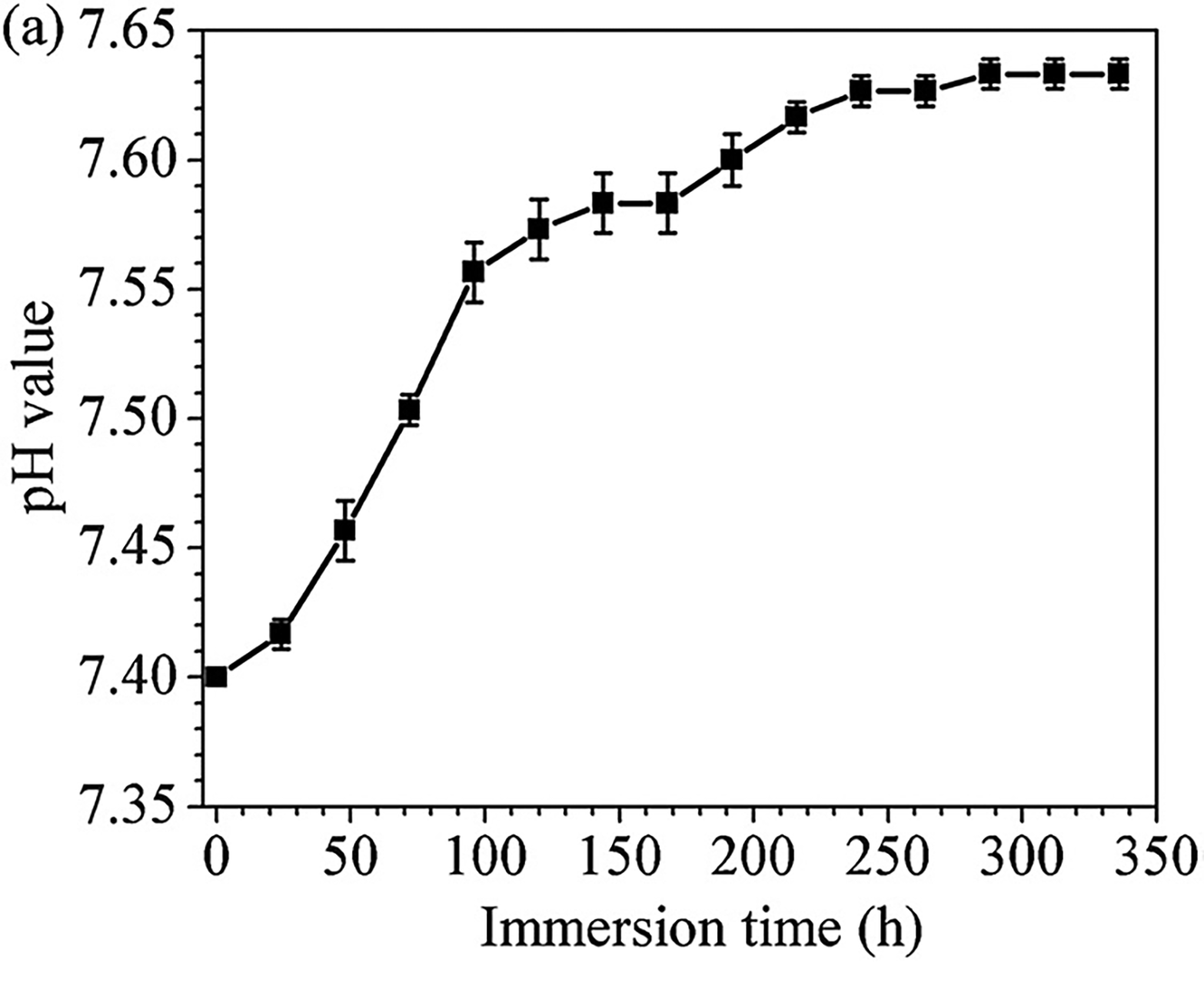
作为浸泡时间函数的 r-SBF 的 pH 值如图 10(a)所示。 r-SBF 溶液的 pH 值在最初浸泡 96 小时期间呈线性增加。 之后通过从 96 小时到 240 小时的进一步浸泡观察到 pH 值的低增加。 然后检测到一个相对恒定的 pH 值 7.63。 如图 10(b) 所示,Zn2+ 的释放速度在 72 小时浸渍内很快,在进一步浸渍过程中变慢。 312 小时后,累积的 Zn2+ 释放 约为 40 mg L-1。 如图 10(c) 所示,Zn 样品的重量损失随时间稳定增加并在 336 小时后达到 1.64 mg cm-2,确定纯 Zn 在 r-SBF 中的永久腐蚀。
The pH values of r-SBF as a function of immersion time are shown in Fig. 10(a). A linear increase in pH of the r-SBF solution was found during the initial immersion up to 96 h. Afterwards as low increase of pH was observed by further immersion from 96 h to 240 h. And then a relatively constant pH value of 7.63 was detected. As depicted in Fig. 10(b), the release rate of Zn2+ was fast within the 72 h immersion and became slow during further immersion. After 312 h, the accumulated Zn2+ release was about 40 mg L−1. As shown in Fig. 10(c), the weight loss of Zn samples increased steadily with time and reached 1.64 mg cm−2 after 336 h, ascertaining the perpetual corrosion of pure Zn in the r-SBF.