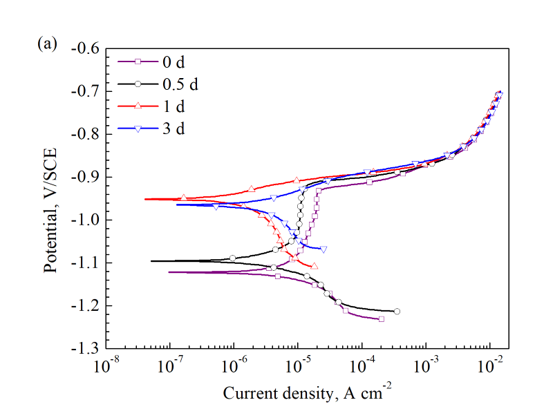
在图 13a 中,检测到阳极分支中的钝化行为(低于 -0.92 V/SCE)持续 0 d 和 0.5 d 样品并在更长的浸泡时间后消失,这可能是由于 BSA 在浸泡和随后解吸开始时的吸附。 浸泡 5 天后,出现小的钝化区域(图 13b)。 这种行为可能与浸泡过程中金属氧化物和 BSA 在样品表面的积累有关。 还可以看出,相同电位下的阳极和阴极电流密度在浸泡 1 d 内大大降低,然后在 3 d 内观察到明显增加。 然而,电流密度在 7 天后再次开始降低。
In Fig. 13a,a passivation-like behavior (below −0.92 V/SCE) in the anodic branch is detected for 0 d and 0.5 d samples and vanishes after longer immersion time, which may result from the adsorption of BSA onset of the immersion and subsequent desorption. After 5 d immersion, small passivation area emergences (Fig. 13b). This behavior could be related to the accumulation of metal oxides and BSA on the sample surfaces during the immersion. It also can be seen that the anodic and cathodic current densities at the same potential reduce greatly within 1 d immersion, then obvious increases are observed over 3 d. However, the current densities start to reduce again after 7 d.