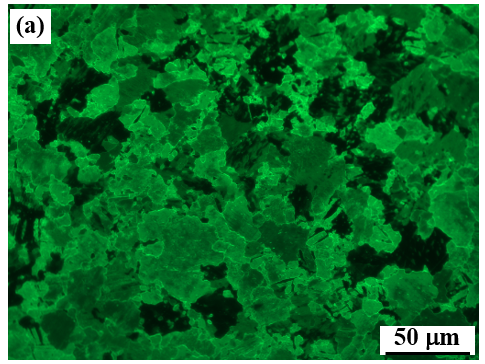
浸泡 1 天后,大部分样品表面被 BSA 覆盖,呈浅绿色。在接下来的 2 天浸渍期间(图 3c),纯 Zn 的几乎整个表面都显示出绿色,表明更多的 BSA 在表面上扩散。值得注意的是,晶界呈现出更亮的绿色,这意味着更多的 BSA 在晶界吸收。延长浸泡时间后,样品表面呈现明显的绿色,晶界变得模糊(图 3d-h)。这些变化表明在浸泡过程中 BSA 在样品表面积累。还需要注意的是,有一些小区域(图 3 中的白色箭头标记)显示强度较低的绿色,甚至在浸泡 28 d 后变为暗区,这是由 BSA 吸附量的反差造成的在表面上。这种现象可能是因为这些区域是溶解在溶液中的优先位点,阻碍了 BSA 的吸附。另一方面,BSA初始吸附后的构象变化也会影响分子间作用力和与样品表面的进一步相互作用。
After 1 d immersion, large parts of sample surfaces are covered by BSA, which shows light green colour. During the next 2 d of immersion (Fig. 3c), almost the whole surface of pure Zn shows the green colour, indicating more BSA spreads on the surface. It is worth noting that the grain boundaries show brighter green colour, which means more BSA absorb on the grain boundaries. On prolonging the immersion time, the sample surfaces display evident green colour and the grain boundaries become obscure (Fig. 3d–h). These changes suggest the accumulation of BSA on sample surfaces during the immersion. It also should be noted that there are some small areas (marked by white arrows in Fig. 3) showing the green colour with low intensity and even change into dark areas after 28 d immersion, which is caused by the contrast of adsorption amount of BSA on the surface. This phenomenon maybe because that these areas are the preferential sites to dissolve in the solution, which hinders the BSA adsorption. On the other hand, the conformation change after initial adsorption of BSA also affects the intermolecular forces and further interaction with sample surface.