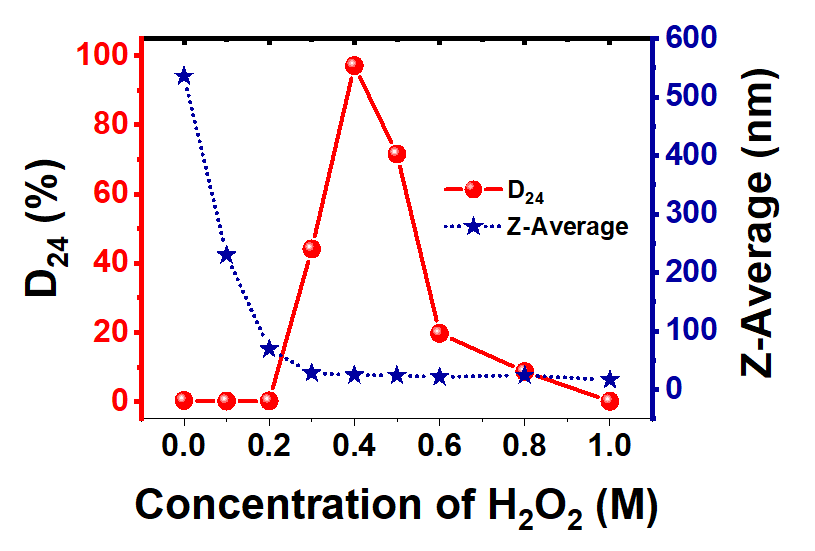
不同浓度H2O2制备的ZrO2纳米颗粒的平均粒径和胶体稳定性(D24)。图中显示了ZrO2浓度对经碱式过氧化氢处理的氧化锆性能影响的结果。Z-平均粒径随H2O2浓度的增加而迅速减小,最高可达1 M。当H2O2浓度为0.3 M时,氧化锆纳米粒子的Z-平均值已降至28.20 nm,随着H2O2浓度的进一步增加,Z-平均值没有相应减小,表明溶液接近饱和影响了H2O2与氧化锆表面的接触。随着H2O2浓度的增加,胶体稳定性先急剧增加后下降。这意味着如果有一些残留的过氧化氢分子,也会影响氧化锆纳米粒子分散体的稳定性。
Z-average particle size and colloidal stability (D24) of ZrO2 NPs prepared with different concentrations of H2O2. The figure shows the results of the effect of ZrO2 concentration on the properties of zirconia treated with basic hydrogen peroxide. The Z-average particle size decreases rapidly with the increase of H2O2 concentration, up to 1 M. When H2O2 concentration is 0.3 M, the Z-average value of zirconia nanoparticles decreases to 28.20 nm. With the further increase of H2O2 concentration, the Z-average value does not decrease correspondingly, indicating that the solution near saturation affects the contact between H2O2 and zirconia surface. With the increase of H2O2 concentration, the colloid stability increases sharply first and then decreases. This means that if there are some residual hydrogen peroxide molecules, it will also affect the stability of the zirconia nanoparticle dispersion.