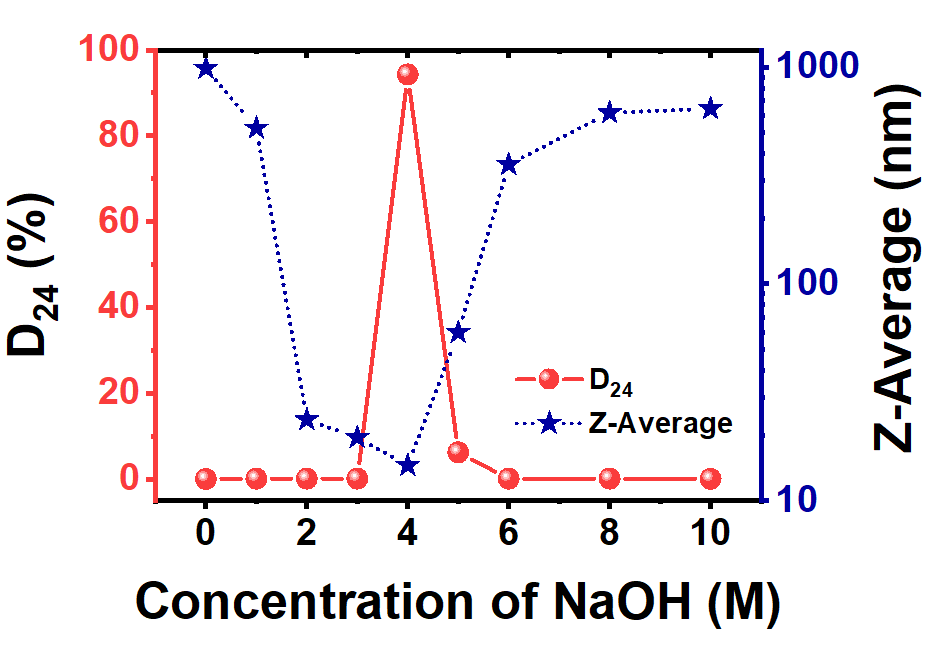
不同浓度NaOH制备的ZrO2纳米颗粒的平均粒径和胶体稳定性(D24)随着NaOH浓度的增加,z -平均粒径先减小后增大;随着NaOH浓度的增加,胶体稳定性先增大后减小;结果发现,NaOH浓度过低或过高都会引起ZrO2的严重团聚。由于在酸洗过程中,H离子在氧化锆表面形成一层电双层,ZrO2能够形成稳定的分散体。整个ZrO2带正电,电荷的排斥作用形成稳定的分散。因此,氧化锆的分散程度也应由氧化锆表面的H离子浓度决定,H离子浓度取决于氧化锆表面的羟基化程度
Z-average particle size and colloidal stability (D24) of ZrO2 prepared with different concentrations of NaOH. As shown, the Z-average particle size decreasedfirst and then increased with an increase of NaOH concentration up to 10 M. The colloidal stability increased dramaticallyfirst then decreased with an increase of NaOH concentration. The results found that the concentration of NaOH is too low or too high, which will cause serious agglomeration of ZrO2. ZrO2can form a stable dispersion because during the pickling process, H ions form an electric double layer with the surface of the zirconia. The whole ZrO2 are positively charged, and the repulsive action of the charge forms a stable dispersion. Therefore, the degree of dispersion of zirconia should also be determined by the concentration of H ions on the surface, which depending on the degree of hydroxylation of the zirconia surface