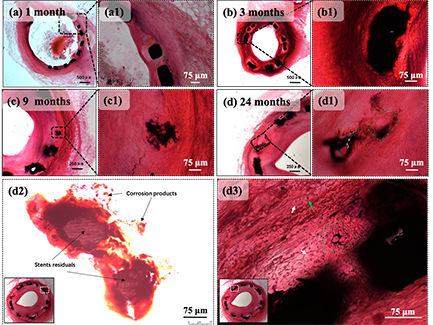
图 5-6 选定节段在以下时间点的H&E染色截面
(a)1个月、(b)3个月、(c)9个月和(d)24个月;(a1)、(b1)和(c1)分别是(a)、(b)和(c)的部分放大图像;(d1)、(d2)和(d3)是(d)的部分放大图像;(d1)绿色正方形标记支柱的先前位置,已被平滑肌细胞取代。(d2)过度曝光获得的图像清晰可见支柱残留物和腐蚀产物(以黑色箭头标记);(d3)白色箭头标记上皮样细胞,绿色箭头标记淋巴细胞,绿色圆圈突出显示异物型巨噬细胞,腐蚀产物大小为10~15 μm
早期研究中发现,在铁基植入物在植入血管9个月后,产生分层的腐蚀产物,并将周围的生物组织推离原始植入物表面>750 μm,几乎没有组织整合[33]。而在图5-6中,整个研究周期内锌铜支架杆残留物和腐蚀产物的周围都被组织紧密围绕,这说明锌铜合金支架具有良好的组织相容性。
图5-6(a)和5-6(a1)可见,内皮化过程可在手术后的第一个月内完成,这很好的印证了OCT观察的结果。快速内皮化是支架术后具有良好的重要条件之一,锌铜支架植入段血管的内皮化速度能够满足临床需求。
Zn-Cu支架植入3个月后,可见支架杆周围聚集有细胞核深染的上皮细胞样巨噬细胞(图5-6(b)和图5-6(b1)),这是异物性炎症过程中较为常见的一种淋巴细胞,是因巨噬细胞吞噬一些不能被消化的细菌或受到其它抗原物质的长期刺激转化而来的[34]。说明锌及及其初级、次级代谢产物作为异物存在于血管壁中,持续刺激机体发生较为平稳的异物性炎症反应。植入9个月后巨噬细胞浸润减少,并转变为轻度炎症(图5-6(c)和图5-6(c1))。在整个观察期内,本研究的锌铜支架的炎性反应情况与纯锌支架在兔模型中的炎症反应相似[35]。本研究未发现添加铜引起的支架血管相关的新风险。
在图5-6(d)显示了部分降解的支架杆,其降解部分已经替换为类似于天然平滑肌细胞(SMC,Smooth Muscle Cell)的细胞所取代。图5-6(d1)中由绿色正方形标记的区域是部分降解的支撑杆的先前位置,该段支架杆已被SMC取代,并且没有严重的炎症反应发生。在原支架杆位置附近观察到了黑色深染的腐蚀产物,腐蚀产物的扩散方向为逆管腔中心方向。图5-6(d2)是通过过度暴光获得的光镜照片,其中黑色箭头标记了支杆的降解产物。图5-6(d2)证明,降解产物在支杆周围形成后,可以分解成小尺寸的产物碎屑(<20 μm)并向外膜的方向扩散(逆向血管管腔),而不是未滞留在原位或者团聚成为不易扩散和进一步降解的大尺寸团聚物。
腐蚀产物的向外迁移有两种可能性。一种是通过细胞间途径中的体液,另一种是通过细胞路径中的细胞吞噬。图5-6(d3)中的绿色圆圈标记了一片腐蚀产物和将腐蚀产物吞噬的细胞。该细胞存围绕腐蚀产物,散乱存在10个以上的细胞核,这是异物巨细胞的形态特征。异物巨细胞(foreign body-type giant cells)是上皮样巨噬细胞受到趋化因子刺激,聚集在锌及其降解产物周围,并进一步联合形成的。图5-6(d3)显示,形成的多核异物巨细胞吞噬了锌铜支架的降解产物碎片,并朝向外膜方向进行转运。这是锌铜支架的降解产物经由细胞途径完成向外膜方向迁移的直接证据,提示锌铜支架降解产物并可能经由人体清除异物系统完成最终代谢。产物想外膜方向转移保证了在这一过程中,支架杆将被正常组织取代,而不会有进入血液循环系统并引起血栓形成或炎症反应的风险。在研究期间,OCT观察到的新生内膜光滑,没有发现血栓形成或严重的炎症反应,也证实了锌铜支架腐蚀产物在支架模型中的转运是安全的。
Figure 5-6 h & E staining section of selected segments at the following time points
(a) 1 month, (b) 3 months, (c) 9 months and (d) 24 months; (A1), (B1) and (C1) are partially enlarged images of (a), (b) and (c), respectively; (D1), (D2) and (D3) are partially enlarged images of (d); (D1) the green square marks the previous position of the strut and has been replaced by smooth muscle cells. (D2) the image obtained by overexposure clearly shows the pillar residues and corrosion products (marked with black arrows); (D3) white arrow marks epithelioid cells, green arrow marks lymphocytes, green circle highlights foreign body macrophages, and the size of corrosion products is 10 ~ 15 μ m
In early studies, it was found that iron-based implants produced layered corrosion products 9 months after implantation of blood vessels, and pushed the surrounding biological tissue > 750 away from the surface of the original implant μ m. There is little organizational integration [33]. In Figure 5-6, the zinc copper alloy scaffold rod residues and corrosion products are closely surrounded by tissues throughout the study cycle, which shows that the zinc copper alloy scaffold has good histocompatibility.
Figures 5-6 (a) and 5-6 (A1) show that the endothelialization process can be completed within the first month after operation, which well confirms the results observed by Oct. Rapid endothelialization is one of the important conditions for good after stent implantation. The endothelialization rate of zinc copper stent can meet the clinical needs.
Three months after the implantation of Zn Cu scaffold, epithelial like macrophages with deeply stained nuclei (Fig. 5-6 (b) and Fig. 5-6 (B1)) can be seen gathered around the scaffold rod. This is a common lymphocyte in the process of foreign body inflammation, which is transformed by macrophages phagocytizing some indigestible bacteria or long-term stimulation of other antigenic substances [34]. It shows that zinc and its primary and secondary metabolites exist in the vascular wall as foreign bodies, which continuously stimulates the body to produce a relatively stable foreign body inflammatory reaction. After 9 months of implantation, macrophage infiltration decreased and changed to mild inflammation (Fig. 5-6 (c) and Fig. 5-6 (C1)). Throughout the observation period, the inflammatory response of zinc copper stent in this study was similar to that of pure zinc stent in rabbit model [35]. In this study, no new risks related to stent vessels caused by copper addition were found.
Fig. 5-6 (d) shows a partially degraded scaffold rod, which has been replaced by cells similar to natural smooth muscle cells (SMC). The area marked by the green square in Figure 5-6 (D1) is the previous position of the partially degraded support rod, which has been replaced by SMC, and there is no serious inflammatory reaction. The black deeply stained corrosion products were observed near the original support rod, and the diffusion direction of the corrosion products was opposite to the center of the lumen. Figure 5-6 (D2) is a light microscopic photograph obtained by excessive exposure, in which the black arrow marks the degradation product of the strut. Figure 5-6 (D2) shows that after the degradation product is formed around the support rod, it can be decomposed into small product debris (< 20) μ m) And diffuse to the outer membrane (reverse to the vascular lumen), rather than not staying in situ or agglomerating into large-size agglomerates that are not easy to diffuse and further degrade.
There are two possibilities for outward migration of corrosion products. One is through body fluid in the intercellular pathway, and the other is through cell phagocytosis in the cellular pathway. The green circle in Figure 5-6 (D3) marks a piece of corrosion product and the cells that swallow the corrosion product. There are more than 10 nuclei scattered around the corrosion products, which is the morphological feature of foreign giant cells. Foreign body type giant cells are formed by epithelioid macrophages stimulated by chemokines, gathered around zinc and its degradation products, and further combined. Figure 5-6 (D3) shows that the formed multinuclear foreign body giant cells phagocytized the degradation product fragments of zinc copper scaffolds and transported towards the outer membrane. This is the direct evidence that the degradation products of zinc copper scaffolds migrate to the outer membrane through the cellular pathway, suggesting that the degradation products of zinc copper scaffolds may complete the final metabolism through the human body's foreign body removal system. The transfer of the product to the outer membrane ensures that in this process, the stent rod will be replaced by normal tissue without the risk of entering the blood circulation system and causing thrombosis or inflammatory reaction. During the study period, the neointima observed by OCT was smooth, no thrombosis or serious inflammatory reaction was found, which also confirmed that the transport of corrosion products of zinc copper stent in the stent model was safe.