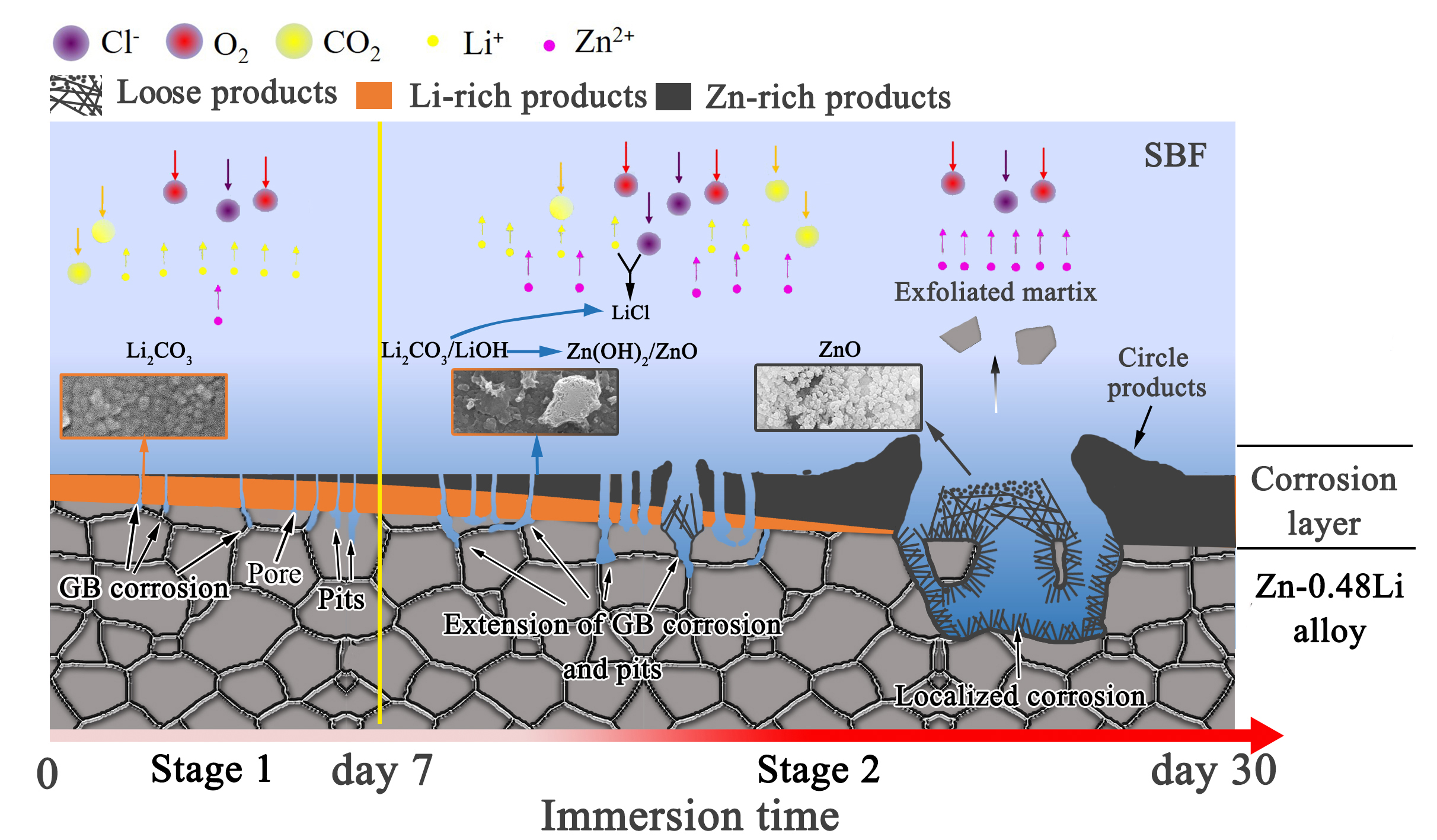
如图13所示,根据上述结果和CRLPR值,腐蚀层在30天内的演化可大致分为两个阶段,如下(一)第一阶段包括前7天。CRLPR值首次从25.3下降微米/年,第1天–14.2微米/年,然后增加到66微米/年,第7天。等离子体显示板和电化学阻抗谱结果表明,厚度为0.2微米形成于表面,主要由非水Li2CO3和少量的Zn(OH)2组成的富锂腐蚀层。也就是说,在SBF浸泡期间,Li+在Zn2+之前释放。这是因为锂在腐蚀层中表现出更大的迁移性和活性,容易与水环境反应,在外腐蚀层中形成含锂化合物。根据XPS(图9和10),水不溶性富锂松香产品和Zn (OH)2根据以下反应形成: 2Li + 2H2O → 2LiOH(s) + H2↑,2LiOH(s) + CO32−→ Li2CO3(s) + 2OH−,Zn + O2+H2O → Zn(OH)2(s)。(二)在第二阶段,CRLPRfirst迅速增加到63.8微米/年,几乎是第一阶段的1倍。电化学测试和体外浸泡的综合结果表明,增厚腐蚀层的保护作用减弱,加速了点蚀的扩展和局部腐蚀的发展(图7(d-h))。重要的是,XPS结果显示腐蚀产物的主要成分变成氧化锌,其从氢氧化锌脱水如下Zn(OH)2(s) → ZnO(s) + H2O
As shown in Figure 13, according to the above results and the CRLPR value, the evolution of the corrosion layer within 30 days can be roughly divided into two stages, as follows (1) The first stage includes the first 7 days. The CRLPR value decreased from 25.3 μm/year for the first time, on day 1-14.2 μm/year, and then increased to 66 μm/year, on day 7. The results of plasma display panel and electrochemical impedance spectroscopy show that the thickness is 0.2 μm is formed on the surface, a lithium-rich corrosion layer mainly composed of non-aqueous Li2CO3 and a small amount of Zn(OH)2. That is, during SBF soaking, Li+ is released before Zn2+. This is because lithium exhibits greater mobility and activity in the corrosion layer, and easily reacts with the water environment to form lithium-containing compounds in the outer corrosion layer. According to XPS (Figure 9 and 10), the water-insoluble lithium-rich rosin product and zinc (OH) 2 are formed according to the following reaction: 2Li + 2H2O → 2LiOH(s) + H2↑, 2LiOH(s) + CO32−→ Li2CO3(s) + 2OH−, Zn + O2+H2O → Zn(OH)2(s). (2) In the second stage, CRLPRfirst rapidly increased to 63.8 μm/year, almost double that of the first stage. The combined results of electrochemical testing and in vitro immersion show that the protective effect of the thickened corrosion layer is weakened, which accelerates the expansion of pitting corrosion and the development of local corrosion (Figure 7(d-h)). Importantly, the XPS results show that the main component of the corrosion product becomes zinc oxide, which is dehydrated from zinc hydroxide as follows: Zn(OH)2(s) → ZnO(s) + H2O