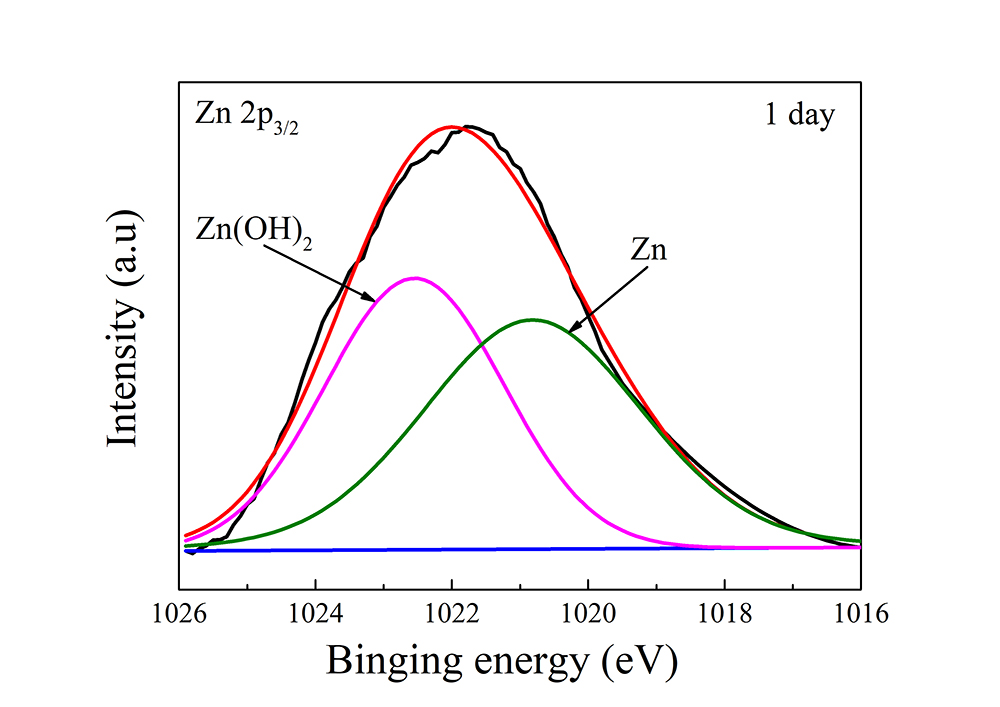
收集了典型样品表面上的Zn 2p 3/2和Li 1s的高分辨率XPS光谱(图10),相应的结果列于表4。第一天的锌2p 3/2p漏与1020.8电子伏和1022.6电子伏的两个贡献非常吻合。前者归属于锌,后者归属于氢氧化锌。浸泡15天和30天后,锌2p 3/2峰的拟合结果显示富锌腐蚀产物为氧化锌(图10(b)和(c))。锂1s光谱的反卷积(图10(d–f))显示富锂腐蚀产物为氯化锂、氢氧化锂和碳酸锂,分别以55.8电子伏、54.9电子伏和55.2电子伏为中心。浸泡一天后,富锂腐蚀产物为Li2CO3(图10(d))。随着浸泡时间的延长,在第5天检测到LiCl(图10(e)),而在第15天检测到LiOH(图10(f))
Deconvolution of Li 1s spectra (Fig. 10(d–f)) reveals that Li-rich corrosion products are LiCl, LiOH and Li2CO3, centered at 55.8 eV, 54.9eV and 55.2 eV, respectively. After immersion for one day, the Li-rich corrosion product is Li2CO3(Fig. 10(d)). With prolonged immersion time, LiCl is detected at day 5 (Fig. 10(e)), while LiOH is detected at day 15 (Fig. 10(f))