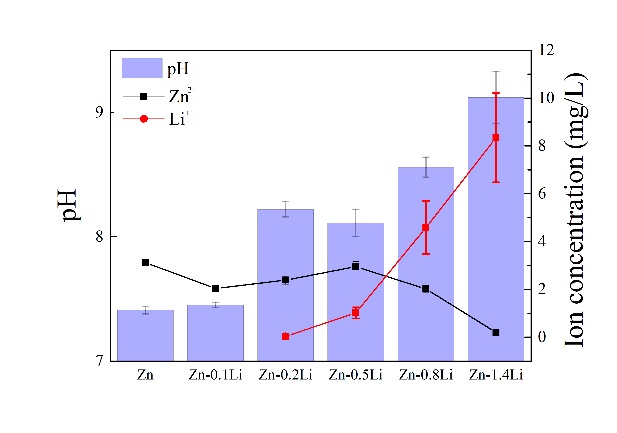
在SBF中浸泡1天后,Zn2+浓度随着Li含量的增加而降低,但Zn-0.5Li合金略有上升,从纯锌的3.12mg/L下降到Zn-1.4Li合金的0.47mg/L(图7a)。同时,Li+浓度随着Li含量的增加增加,从Zn-0.2Li合金的0.04mg/L到Zn-1.4Li合金的8.35mg/L(图7a)。当Li>为0.5%时,Zn2+浓度低于Li+浓度的关系保持不变。对于Zn-0.8Li合金,其Li+/Zn2+原子比约为21,对于Zn-1.4Li合金急剧上升至约388(图7b)。这表明,在阳极腐蚀反应中,Li+释放剂超过Zn2+释放。结果表明,pH值随Li含量增加,归因于Li与水反应释放的氧化氢离子。
After immersion in SBF for 1 day, Zn2+ concentration decreases with Li content, except a slight rise for Zn-0.5Li alloy, from 3.12 mg/L for pure Zn down to 0.47 mg/L for Zn-1.4Li alloy (Fig. 7a). Meanwhile, Li+ concentration increases with Li content, from 0.04 mg/L for Zn-0.2Li alloy up to 8.35 mg/L for Zn-1.4Li alloy (Fig. 7a). The relation of Zn2+ concentration lower than Li+ concentration on longer keeps when Li > 0.5%. For Zn-0.8Li alloy, its Li+/Zn2+ atomic ratio is about 21, which rises dramatically to about 388 for Zn-1.4Li alloy (Fig. 7b). This demonstrates that Li+ releasement dominants over Zn2+ releasement in anode corrosion reaction. It is demonstrated that pH value increases with Li content, attributing to OH-ions released from the reaction of Li with H2O.